

In a few seconds how small of a scale it is, we're going to have to have So if we're going to operateĪt this small of a scale, and we're gonna appreciate Predictions about what will happen, and make things that are
We can then begin to understand the universe in which we live in, the scale in which we live in, and even be able to make Thinking about the atomic, and even the subatomic scale. That I've always found amazing about chemistry, it's an entire field of science that we as human beings have developed to actually understand what is happening in an almost unimaginably small scale.
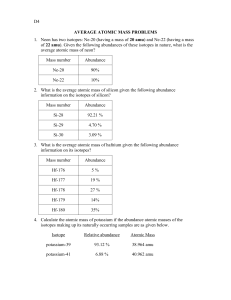
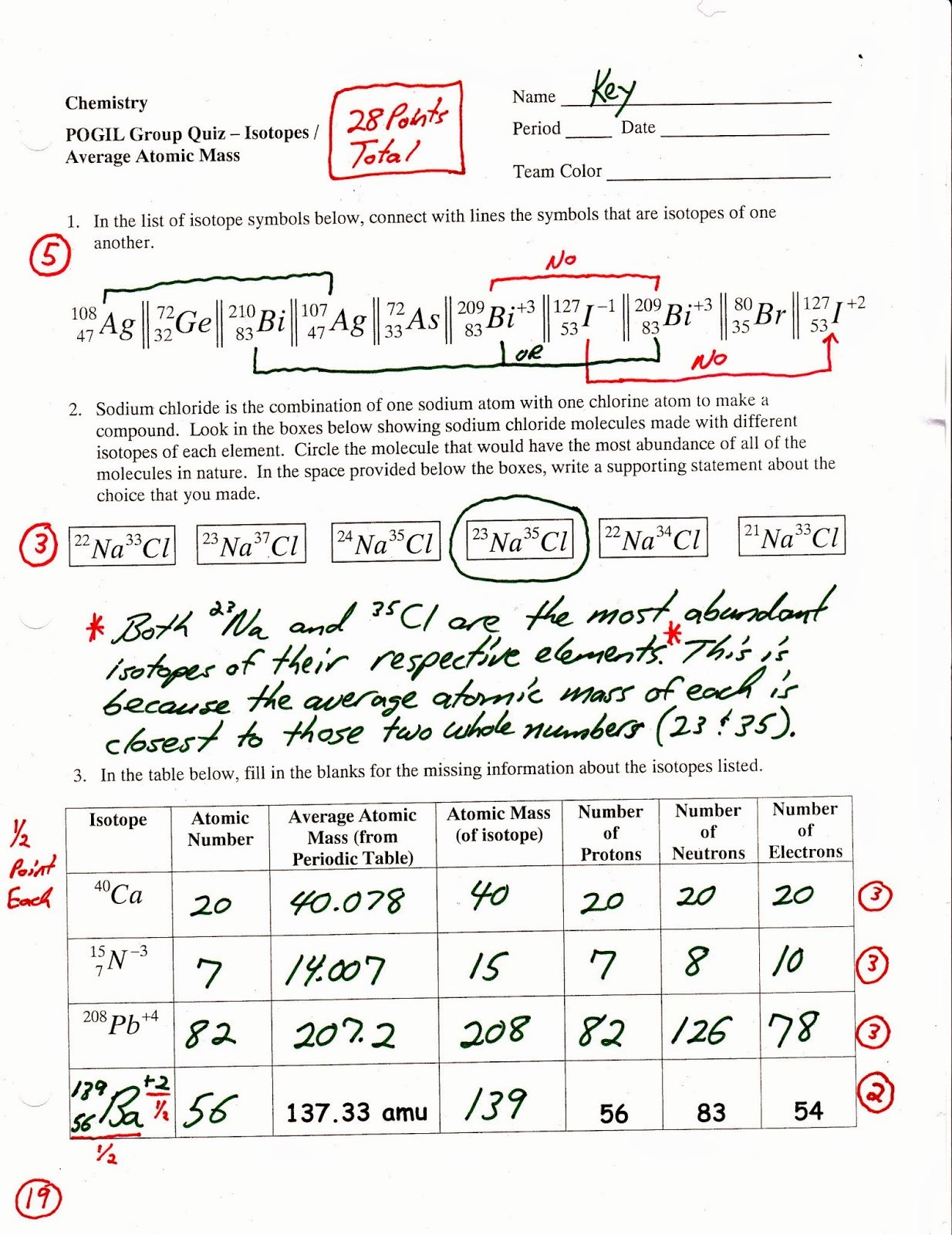
Keep in mind for percentages to be used in a calculation like this they need to be in decimal form. For this calculation we multiply the masses by their percentages and sum them up to find the weighted average. This means that certain isotope's atomic masses contribute more to the element's average mass than other isotopes.įor the hypothetical element Sal is describing, 80% of the element's atoms have an atomic mass of 5 amu, while only 20% have an atomic mass of 6. For average atomic mass, the isotopes of an element are found in difference abundances or amounts in nature. The difference between the two types of averages is that an arithmetic average are of equal importance, but a weighted average takes into account the importance of some values over others. But for average atomic mass we're calculating a weighted average. It we wanted the arithmetic average then it would be 5.5 because (5+6)/2 = 5.5. I hope this helps! Wherever you are in the world, I wish you luck in chemistry and whatever courses you will be taking in the future! But I'm still wondering about how many versions there can be for an element. My question: How many "versions" are there for each element? Is there an infinite # of versions? And if so, how do you calculate that average (aam)?Įdit: Actually, the next video goes into calculating the aam. Isotopes - same element w/ different # of neutrons. ★ The number under the elements in the periodic table (for example, the "1.008" in Hydrogen) is the "aam", or average atomic mass.Īverage atomic mass - weighted average of various versions of the element (ex. what a number!)Įlectron - almost one two thousandth of a proton or neutron (in other words, really small!)
Average atomic mass problems worksheet free#
If I got anything wrong, feel free to comment below (I would appreciate it)!Ītomic mass unit (or "amu") - also known as "u", or "unified atomic mass unit". Hi! Here are some brief notes that I took in the video. Also, scientists needed a pure isotope to base the system on. Scientists used carbon-12 because no other atom has exact whole-number masses in the amu scale. This is why the mass of carbon-12 is 12 amu, not 12.09. Some of the mass of the atom gets converted into binding energy, so the mass of the entire nucleus would actually be less than the mass of each individual proton and neutron added together. Because of energy-mass equivalence (E = mc^2), we know that energy and mass are interchangeable. Binding energy is the energy that holds the nucleus together.
Average atomic mass problems worksheet full#
Since the nucleus is full of protons, which has positive charge, they would repel and the nucleus would fly apart. The missing mass is called mass defect, and it represents the binding energy. Following this logic, the carbon-12 atom's atomic mass should actually be 12.09 amu however, the mass is exactly 12 amu. So the mass of a proton is around 1.008 amu, not 1. The relative abundance of Deutrium (1 proton, 1 neutron) is so small that it is barely accounted for when calculating the average atomic mass. Protium is by far the must abundant isotope of hydrogen, and it only contains 1 proton, and no neutrons. The average atomic mass for hydrogen is actually around 1.008 amu. But after isotopes were discovered (oxygen-17, oxygen-18, etc), everything got really confusing, so scientists agreed to use carbon-12. \)).Good question that requires a kind of long explanation!Ī long time ago, the standard for measuring average atomic mass was actually based on oxygen, and scientists thought all oxygen was oxygen-16 (8 protons, 8 neutrons).


 0 kommentar(er)
0 kommentar(er)
